Acute lymphoblastic leukemia (ALL) in adults has been historically associated with dismal clinical outcomes. Fortunately, adults diagnosed with ALL are now more likely to experience a favorable response to treatment due to ongoing improvements in immunotherapy.
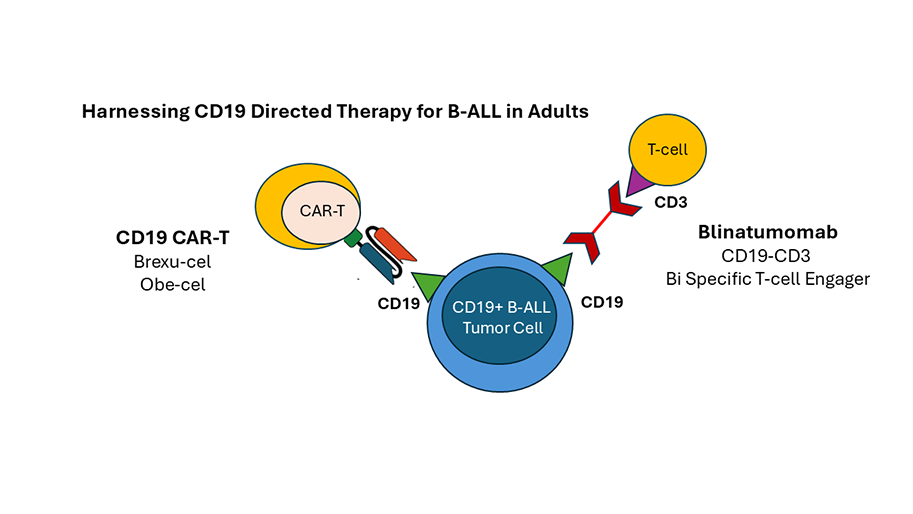
The most significant advance in the treatment of ALL in adults has been the development of blinatumomab, a CD19-CD3 bi-specific T-cell engager (BiTE) for treatment of B-ALL. Blinatumomab was the first immunotherapy approved for B-ALL, when it was demonstrated in the randomized, phase 3 TOWER study to be superior to conventional salvage chemotherapy for relapsed and refractory B-ALL. Subsequent study of blinatumomab for persistent and recurrent measurable residual disease (MRD) revealed notably higher response rates, underscoring the forte of this agent in the setting of low disease burden. This inspired the National Clinical Trials Network (NCTN) of the National Cancer Institute (NCI) to launch the E1910 trial which randomized adult patients with Philadelphia chromosome-negative B-ALL in MRD-negative CR to receive conventional chemotherapy or conventional chemotherapy plus four cycles of blinatumomab during the consolidation phase. The E1910 trial, which the Adult Leukemia Program at Dana-Farber Cancer Institute participated in, demonstrated that the addition of blinatumomab to consolidation chemotherapy significantly improved overall survival (OS) leading to FDA approval for this indication.
Current efforts are looking to capitalize on the success of blinatumomab consolidation by de-escalation of chemotherapy (ECOG 1910 maintained same chemotherapy backbone in both groups) with hope of maintaining efficacy while decreasing toxicity. Multiple studies are exploring this concept in different patient populations, including an effort led by Marlise R. Luskin, MD, MSCE, and Daniel J. DeAngelo, MD, PhD, at Dana-Farber to treat older adults with a venetoclax-based low intensity induction followed by blinatumomab consolidation. Initial results of the Phase 1 portion of this study were recently published, while the Phase 2 portion, which focuses on newly diagnosed older adults and incorporates blinatumomab for patients with B-ALL, is nearing accrual with initial promising results.
Blinatumomab is also showing benefit in adults diagnosed with Philadelphia chromosome-positive (Ph+) ALL, a historically aggressive subtype of ALL. As recently reviewed by Luskin, multiple studies are now demonstrating the ability of blinatumomab to replace intensive, toxic chemotherapy and allogeneic hematopoietic stem cell transplant in many patients, allowing patients to be cured entirely without chemotherapy. As recently profiled in this publication, Dr. Luskin is leading an ongoing trial exploring a novel dual TKI approach for treatment of Ph+ ALL, now combined with blinatumomab with the first report of this effort presented at ASCO 2025.
Led by Dr. DeAngelo, Dana-Farber has also participated in the development of autologous CD19 chimeric antigen receptor (CAR) T-cell therapy for adult ALL enrolling patients on the ZUMA-3 study of brexucabtagene autoleucel (approved by the FDA in 2021 as the first CAR T for adult ALL) and the recently published FELIX study of obecabtagene autoleucel, a novel intermediate-affinity anti-CD19 CAR T recently FDA approved in 2024 as the second CAR T therapy for adults with ALL. The FELIX study reported frequent durable responses in adults with R/R B-ALL and – importantly – extremely low rates of cytokine release syndrome and immune effector cell associated neurotoxicity syndrome. The efficacy and safety of CAR T for R/R ALL in adults of all ages is inspiring ongoing studies of incorporating CAR T into frontline consolidation therapy for both Ph- and Ph+ ALL, looking to extend the success of blinatumomab immunotherapy in improving the consolidation phase of treatment for B-ALL. The future is promising for adults with ALL – leukemia investigators at Dana-Farber continue to participate in these exciting efforts.