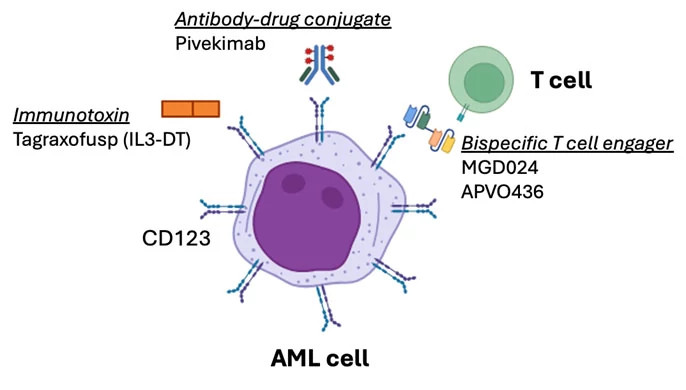
CD123 is the alpha subunit of the interleukin 3 (IL-3) receptor. It pairs with a beta subunit, CD131, to transmit cytokine signals from the outside to inside of several types of immune and hematopoietic cells. CD123 is highly expressed on the cell surface of several blood cancers. Approximately 80% of acute myeloid leukemias (AMLs) express CD123, and the level of CD123 in AML is higher than on normal blood stem cells. CD123 is also a marker of leukemia stem cells, which are a small subset of AML within a given patient that can produce more leukemia cells. Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is an aggressive subtype of AML that has very high levels of CD123 in all patients. CD123 is also expressed in some patients with other blood cancers, including myelodysplastic syndrome (MDS), myeloproliferative neoplasms (MPNs), B-cell acute lymphoblastic leukemia (B-ALL), and others.
Several exciting new drugs have been discovered that target CD123. The first CD123-directed drug was tagraxofusp, a targeted immunotoxin that mimics IL-3 binding to CD123 and delivers a toxic molecule to the leukemia cells. Tagraxofusp is approved for the treatment of BPDCN and is in clinical trials in several other blood cancers as a single-agent or in combination with other leukemia therapies. Members of the Adult Leukemia Program and BPDCN Center at Dana-Farber Cancer Institute led the development of tagraxofusp.
Another way to target CD123 is with an antibody-drug conjugate (ADC), which links a chemotherapy molecule to an antibody and thus enables targeted treatment without causing toxicity in other organs. Pivekimab is a CD123-ADC that has been tested in trials at Dana-Farber for patients with AML and BPDCN, both alone and with other leukemia therapies like azacitidine and venetoclax. Pivekimab received Breakthrough Designation by the FDA for treatment of BPDCN and we are eagerly awaiting a decision on potential approval.
A third way to target CD123 is by recruiting the patient’s own immune system to attack the leukemia cells. There are currently two trials evaluating CD123 x CD3 bispecific antibodies or T cell engagers for patients with CD123-positive AML and other blood cancers that express CD123. A bispecific drug targets two molecules simultaneously, which can bring two different cells into contact with each other. In this case, one side of the drug binds to CD123 on the leukemia cell and the other side binds to CD3 on the patient’s T cells. The recruited T cells can then kill the leukemia cells by an immune attack. Current studies at Dana-Farber are evaluating MGD024 and APVO436, which are both CD123 x CD3 bispecific T cell engagers.
CD123 targeting drugs currently part of standard clinical care at Dana-Farber, and new CD123 drugs recently or currently available in trials at Dana-Farber are:
Tagraxofusp (IL3-diptheria toxin)
- Approved for BPDCN
- Dana-Farber 17-056 [open], PI: Andrew Lane, MD, PhD (NCT03113643): tagraxofusp with azacitidine for untreated or relapsed high-risk MDS
- Dana-Farber 24-479 [open], PI: Andrew Lane, MD, PhD (NCT06456463): tagraxofusp with azacitidine and venetoclax for untreated CD123+ AML
- Tagraxofusp with azacitidine and venetoclax for CD123+ AML in remission but with minimal residual disease (MRD) positivity (opening soon, PI: Jacqueline Garcia, MD)
Pivekimab (CD123-ADC)
- Trials recently completed for BPDCN and CD123+ AML, single agent and combinations; FDA Breakthrough Designation for BPDCN, awaiting regulatory decision (Dana-Farber PIs: Andrew Lane, MD, PhD; Daniel DeAngelo, MD, PhD)
MGD024 (CD123 x CD3 bispecific)
- Dana-Farber 22-482 [open], PI: Eric Winer, MD (NCT05362773): MGD024 for relapsed/refractory hematologic malignancies
APVO436 (CD123 x CD3 bispecific)
- Dana-Farber 25-053 [open], PI: Evan Chen, MD (NCT06634394): APVO436 with azacitidine and venetoclax for newly diagnosed, CD123+ AML
Tagraxofusp is a CD123 targeted immunotoxin approved for BPDCN and currently in trials for AML, MDS, and other blood cancers. Pivekimab is a CD123-antibody drug conjugate that was recently tested in trials at Dana-Farber for patients with BPDCN and AML, both as a single agent and in combination with other chemo and targeted therapy. MGD024 and APVO436 are CD123 x CD3 bispecific T cell engagers that recruit T cells into proximity with AML cells and are both in current clinical trials in the Adult Leukemia Program at Dana-Farber.